Results of the RIDD trial
Background
Dupuytren’s disease is a common condition of the hand that affects about 5% of the UK population. It is an inflammatory condition that causes nodules to grow in the palm, causing the fingers to curl inwards and resulting in significant impairment of hand function.
Currently there is no approved treatment for early stage Dupuytren’s disease. Without treatment patients must wait until the finger has curled into the palm before they can be offered a surgical procedure to relieve the symptoms, but the condition often returns.
The RIDD trial
In our research we found that the cells that produce the excess tissue in the nodules and cords, and cause the tissues to contract, are dependent on Tumor Necrosis Factor (TNF). So, we designed the RIDD trial to see if a drug which inhibits TNF (called anti-TNF) would halt or slow progression of the disease.
In an early trial (called a phase 2a dose-ranging clinical trial), we identified the optimal dose and formulation of adalimumab, the anti-TNF agent we are using.
In the second phase of the trial (phase 2b clinical trial) we randomised participants 1:1 to receive 4 injections at 3 monthly intervals of either adalimumab or saline (placebo). Our primary measure of success was a decrease in nodule hardness (measured using an instrument called a durometer) at 12 months.
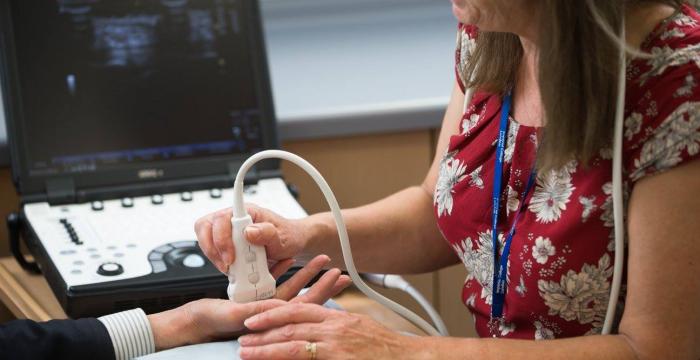
As a secondary measure of success, we looked for a decrease in nodule size on an ultrasound scan. We observed both these measures, with statistically significant differences, and both continued to decrease for 9 months after the fourth injection.
Patients with early stage Dupuytren’s disease have little impairment of hand function and we did not find any changes over the course of the trial. More patients in the saline group progressed or were awaiting surgery compared to the adalimumab group, although the overall numbers were small. The treatment was safe and there were no related serious adverse events.
Taken together, our laboratory, phase 2a and phase 2b clinical trial data suggest that injections of adalimumab directly into the nodules, may control progression of early stage Dupuytren’s disease. However, follow up over approximately 10 years would be required to confirm the long-term effects on finger flexion and hand function. If the nodule were to re-activate we envisage that each active nodule would need to be injected and patients would undergo a further series of injections.
The results of the phase 2b trial have been published in The Lancet Rheumatology and the full paper can be accessed via:
https://doi.org/10.1016/S2665-9913(22)00093-5
or
https://www.thelancet.com/journals/lanrhe/article/PIIS2665-9913(22)00093-5/fulltext
Adalimumab is not approved for treatment of early stage Dupuytren’s disease and was used off label for this trial. The trial team are unable to provide comment on treatment of individual patients, who should seek the advice of their general practitioner or surgeon.